The Mystery Of The Black Line
During pregnancy, many women notice a dark line stretching up the center of the belly, from the pubic bone toward the chest. It’s called the linea nigra, Latin for “black line,” and it’s commonly attributed to hormonal changes¹ ². But that explanation only scratches the surface.This line appears thanks to melanin—not just reacting to hormones, but helping guide them. Far more than a side effect of pregnancy, the linea nigra may be one of the clearest early signs that melanin is doing something most essential: organizing the creation of new life.The Axis Of Assembly
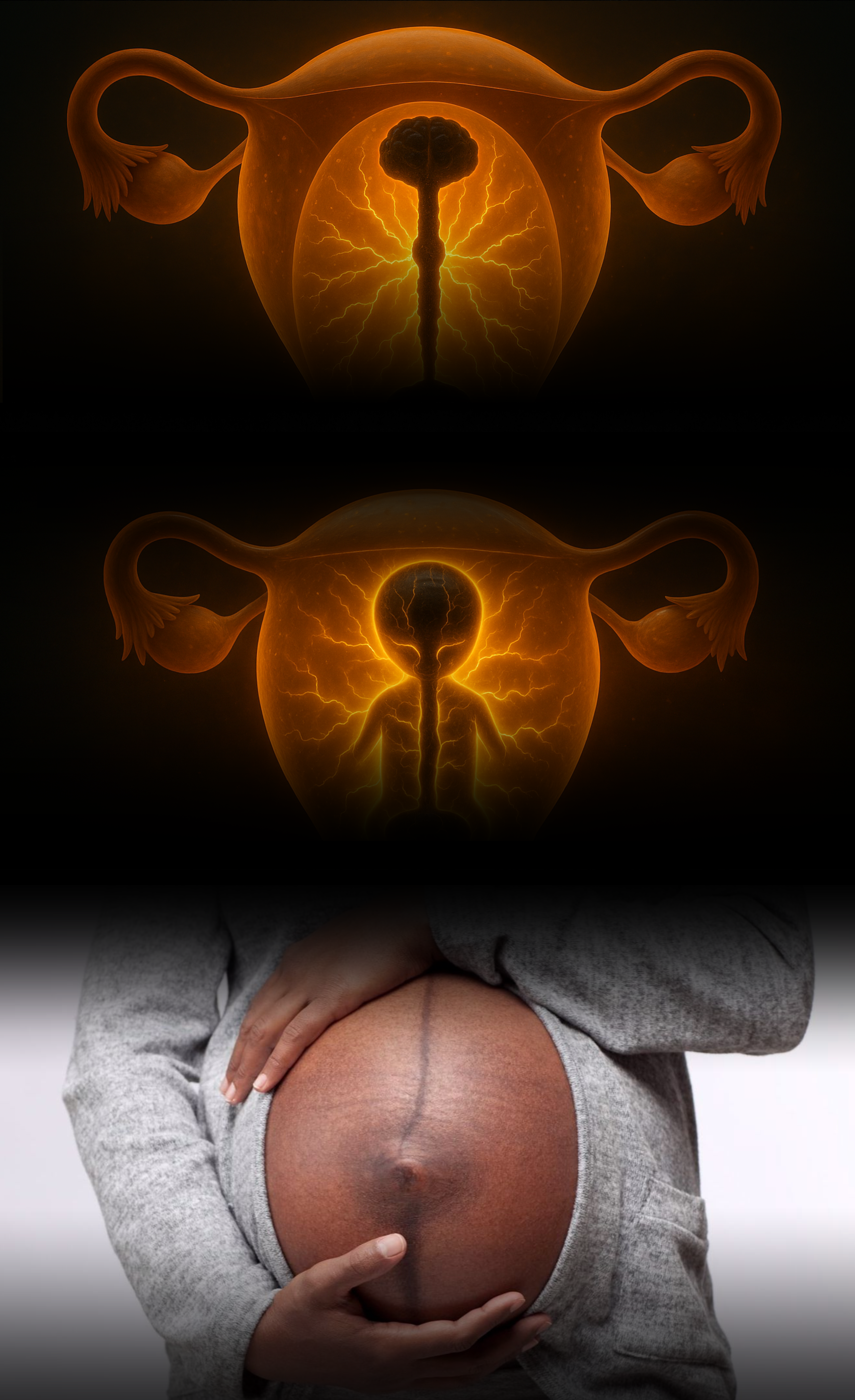
Before the skin forms, before the brain and spine take shape, and long before the embryo resembles a recognizable human form, development begins around a single vertical line. This structure, called the primitive streak, appears along the embryo’s center and is composed of melanin-rich stem cells³ ⁴.
This streak is not temporary, but serves as the central axis from which the entire body develops. From this line, the spine emerges. Then the brain. Then the limbs, organs, and sensory systems³.
Emerging research suggests that this structure coordinates electrical currents; a stream of bioelectrical signals that tell cells where to migrate, when to divide, and what to become⁵ ⁶. In effect, this early streak acts like a biological compass, aligning the entire developmental process along a coherent, central path. And melanin appears to be at the center of it, quite literally. A Mirror Across Bodies
What’s remarkable is that as the streak unfolds, the developing fetus aligns vertically—head to toe—in direct orientation with the mother’s body, meaning the internal line sits directly underneath the linea nigra on the her skin. This creates more than anatomical symmetry. It forms a kind of energetic echo: a shared bioelectric current rising through both mother and child. Melanin marks this invisible link across bodies, mirroring the alignment of life in two forms.A Blind Spot In Science
Most biology textbooks acknowledge the primitive streak. But few, if any, name one of its most striking features: its melanization³ ⁴. Why the omission?Because acknowledging melanin at this stage of development disrupts the conventional narrative. Melanin is still widely framed as a late-stage trait; an evolutionary adaptation for UV protection or cosmetic variation, but little else.But if melanin is present at the very beginning—before skin even forms—then it suggests a bigger role altogether.And yet, many scientific papers use vague terms like “dark cells” or “pigmented lines” instead of naming melanin outright. Language that can blur the focus, and make it easier to overlook what's actually there. Once we recognize melanin’s presence in the earliest embryological structures, we are forced to reconsider its role—not as a byproduct of environmental or external conditions, but as a primary organizer of development—directing pattern, polarity, and growth from the start.The Spark Before The Streak
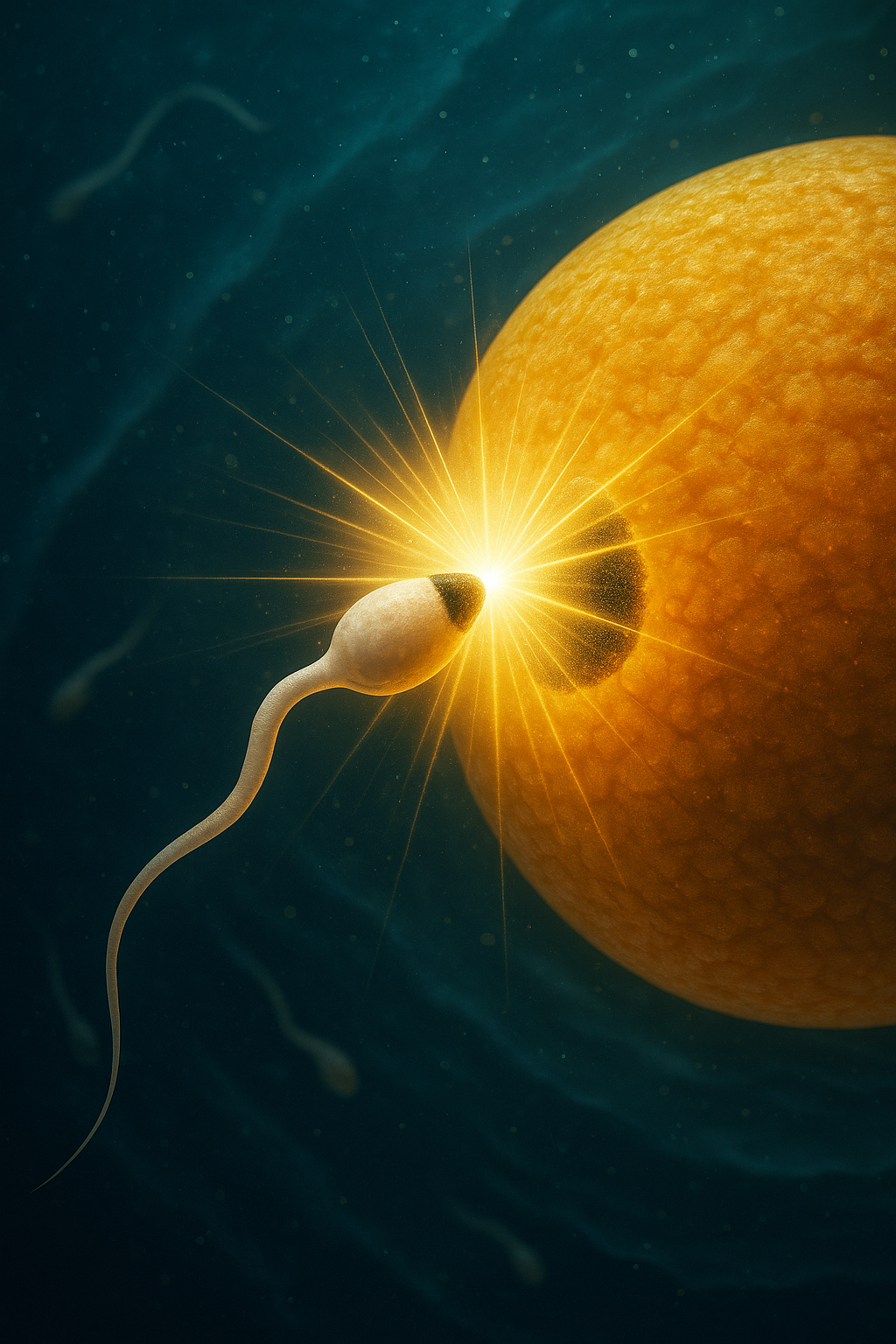
But melanin’s role doesn't begin with the streak. It begins before conception itself.Studies show that it is present on the acrosome, the tip of the sperm that penetrates the egg, and on the outer membrane of the egg, precisely where the sperm enters⁷. These darkened areas are not random but appear to be melanized by design.When sperm and egg meet, a flash of light erupts at the point of contact. This moment, called the “zinc spark”, marks the beginning of fertilization. It is triggered by the regulated release of zinc ions from the egg, resulting in a brief but measurable burst of bioelectricity⁸ ⁹.
Melanin seems to enable this reaction in two key ways:
It carries the electrical charge needed to initiate the spark¹⁰.
It absorbs and stores heavy metals like zinc, regulating when and how they’re released⁸ ⁹.
Even more compelling, the sperm appears to be drawn to the exact melanin-rich spot on the egg, possibly guided by magnetic or electric fields. And melanin, especially when bound to metals like zinc, is known to respond to magnetic fields¹⁰. This opens the possibility that it may play a role not just in the fusion of gametes, but likely in the spatial guidance that leads to that fusion¹¹.The Direction Of Becoming
The linea nigra doesn’t just form. It ascends. It stretches from the pubic bone upward toward the chest, reflecting a universal blueprint: life moves in the direction of elevation.Plants grow toward light. Infants rise from crawling to standing. Heat, energy, and even consciousness all follow a vertical trajectory. Across ancient traditions, this upward movement appears again and again—through rising kundalini, ascending chakras, and the sacred architecture of pyramids—not simply as metaphor, but as descriptors of energetic movements.The linea nigra is a trace of that same principle: life rising. A visible signal of an invisible truth. And melanin, the substance that draws the line, is not merely a detail of that principle. It's the centerpiece.References
Handel, A. C., Miot, L. D. B., & Miot, H. A. (2014). Melasma: A clinical and epidemiological review. Anais Brasileiros de Dermatologia, 89(5), 771–782.
Kim, A. M., Vogt, S., O'Halloran, T. V., & Woodruff, T. K. (2010). Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nature Chemical Biology, 6, 674–681.
Deuchar, E. M. (1958). The Primitive Streak and Notochord. Oxford: Blackwell Scientific.
The enigmatic primitive streak: prevailing notions and challenges. (2016). Developmental Biology Review.
Levin, M. (2014). Bioelectrical controls of morphogenesis: from ancient mechanisms to synthetic bioelectricity. PMC.
Tseng, A. & Levin, M. (2013). Bioelectric mechanisms in regeneration and development. PMC.
Van Duin, M., Polman, J. E., De Rooij, D. G., & Grootegoed, J. A. (1990). Chromatin structure and transcriptional activity of the mouse spermatozoal genome during fertilization. Dev Biol, 141(1), 202–208.
Que, E. L., Bleher, R., Duncan, F. E., Kong, B. Y., et al. (2015). Quantitative mapping of zinc fluxes in the mammalian egg reveals the origin of fertilization-induced zinc sparks. Nature Chemistry, 7, 130–139.
Kim, A. M., Vogt, S., O'Halloran, T. V., & Woodruff, T. K. (2010). Zinc availability regulates exit from meiosis. Nature Chemical Biology, 6, 674–681.
Sarna, T., & Swartz, H. M. (1998). The physical properties of melanin. In Melanins and Melanosomes (pp. 333–377). Wiley-VCH.
Eisenbach, M. & Giojalas, L. C. (2006). Sperm guidance in mammals — an unpaved road to the egg. Nat Rev Mol Cell Biol, 7, 276–285.